How Artificial Intelligence is Shaping FDA’s Thinking
There is a quiet revolution taking place inside the FDA!
Rapid advancement in Artificial Intelligence (AI) is creating tremendous possibilities for innovation in the MedTech industry. As a result, the FDA is evolving its approach to the evaluation of new products for market approval. Using AI, FDA also wants to make faster and better regulatory decisions through targeted inspections of medical device manufacturers for compliance to Quality Systems Regulations.
Recently, I had an opportunity to hear directly from FDA leaders during a fire-side chat at the Xavier Health AI Summit in Cincinnati, OH. Alonza Cruse, Director, FDA - Office of Regulatory Affairs and Bakul Patel, Associate Director, FDA – Center for Devices and Radiological Health, each shared their views on how AI is shaping FDA’s thinking in nearly all of its operations. It was very encouraging to hear that the FDA wants to build a collaborative relationship with the industry and “stand out of your way” to help realize the true potential of AI in Healthcare.
The consensus? This is good news for the industry!
There is a widespread sense that the agency is highly “risk-averse” and requires a ton of data from clinical trials before approving new products. When it comes to an inspection, the notion of the FDA knocking at your facility is enough to get everyone on a high alert in itself. Hearing about a collaborative approach straight from the FDA is encouraging because it will be a catalyst to accelerate innovation, not to mention enabling speed-to-market for innovative products, ultimately increasing benefit to patients.
AI can help the agency gain insights and drive innovation from the vast amount of data.
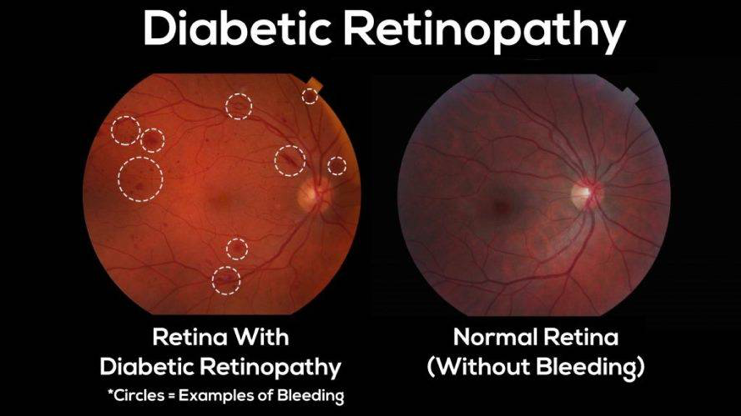
Case in Point: Recently IDx-DR was approved, which is an AI-driven diagnostic system that automatically analyzes images of the retina and gives an alert if the patient has a high likelihood of developing diabetic retinopathy. Nearly 24,000 people lose their vision due to this disease, which can be prevented through early intervention. Needless to say, IDx-DR is a major breakthrough.
Approximately 50 percent of people with diabetes do not get an annual vision exam, where a specialist can easily detect this problem from the retinal images. When IDx-DR technology is used in a Primary Care facility, the doctor or the technician can quickly refer the patient to a specialist based on the alert given by the AI system, thus helping detect diabetic retinopathy early and prevent vision loss.
The FDA designated IDx-DR as a breakthrough device and provided intensive interaction and guidance to the company on efficient device development and data collection for the review process before approval.
When it comes to enforcement, the FDA is taking a risk-based, targeted approach to Quality System compliance. Using AI tools on the vast amounts of adverse events and real-world data, the goal is to drive both efficiency and effectiveness of inspections.
Case Example: The FDA is planning to invest heavily in NEST, a national level data gathering system on medical devices from the real world, including data from patient registries, claims and electronic medical records. This is a public-private partnership which already has access to 495 million patient records from more than 195 hospitals and nearly 4,000 outpatient clinics. Comments from the leader of the FDA clearly indicates their vision of using AI tools to identify anomalies and safety signals from these data sources, and take proactive measures in a systematic, targeted way. They will focus on devices with higher risks or emerging safety signals, as well as on manufacturers who are slow to respond to problems identified during prior inspections. The FDA would also like to increase the efficiency of inspections and make more resources available to support innovation and product approvals.
In a risk-based approach targeting manufacturers of Infusion Pumps, FDA has conducted 496 inspections of 165 manufacturing facilities and issued 40 warning letters during the period 2010-2017.
This change in mindset is driven directly from the top. FDA commissioner Scott Gottlieb has made several public comments about his views on Artificial Intelligence (AI) and Machine Learning (ML), and how the agency is responding to support AI based innovation.
Cause for Applause!
Such a positive and supportive view is an opportunity for the MedTech industry. FDA leaders at the AI Summit encouraged industry to work collaboratively with the agency, and to engage early in the development process. They also encouraged application of AI in improving Quality Systems by emphasizing the “current” in cGMP, and indicating that it is an expectation that practices should evolve with technological advancements.
In the current environment, the question is not whether to use AI tools; rather the question to ask is whether it is irresponsible to not use AI!
References:
Xavier Health AI Summit, 2018
FDA approval notice for IDx-DR, April 2018
IDx-DR, 2018