A Risk Management Tool for MedTech
Objective classification of adverse events has been a challenge for the industry. An outcome-based, medically-focused classification framework offers a more robust approach.
According to ISO 14971, the International Standard for application of risk management to medical devices, the term risk is defined as the “combination of the probability of occurrence of harm and the severity of that harm”. In general, quantitative or semi-quantitative criteria for probability of occurrence of harm are available for risk assessment. However, classification of severity is generally done using subjective terms such as negligible, minor, major, critical and catastrophic. Although considerable effort is spent in creating definitions for these terms, there is inconsistency in application during risk assessments, even by expert medical professionals. What is minor to one, may be major to another based on individual risk perception shaped by personal experience and subject matter expertise.
This problem was recognized by the medical community in the early 1990’s and a simplified grading scale was proposed by Dr. Pierre A. Clavien at University Hospital of Zurich, Switzerland, to rank complications based on the therapy used to treat negative surgical outcomes. In 2004, Dr Clavien and his collaborators further refined the system to a 5-point grading scale and validated their approach with an evaluation in a cohort of over 6000 patients and an assessment of acceptability and reproducibility by medical professionals worldwide.
The resulting Clavien-Dindo Classification system is now widely used, and continues to gain popularity. As the Healthcare payment model moves from fee-based to outcome-based, a more objective, measurable approach is needed to compare outcomes among different healthcare facilities, between therapies and within a healthcare center over time. Even the FDA has shown an interest in this system as a way to assess benefit-risk of medical devices and therapies.
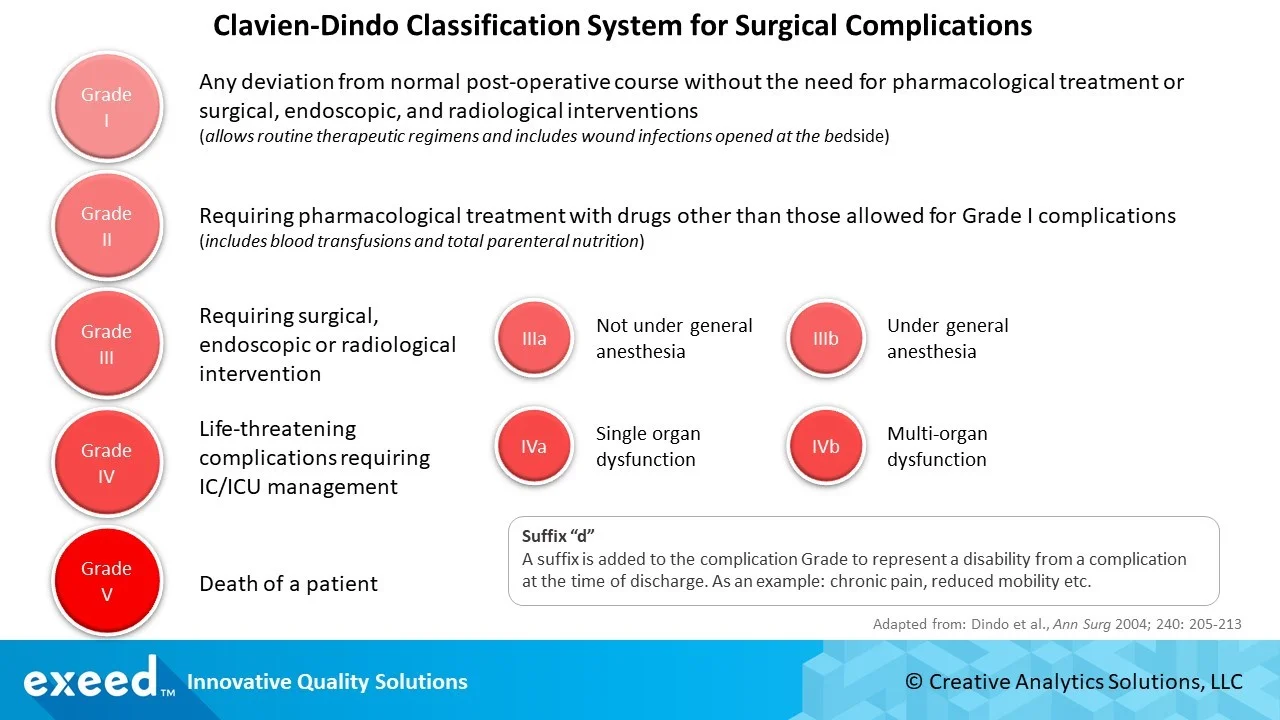
The graphic below shows a summary of the 5-point Classification System. It can be tailored for specific therapies or devices based on their unique characteristics. As an example, all over the counter medications can be included in Grade I and Grade IIIa, IIIb, or Grade IVa, IVb can be contracted in
Grade III and Grade IV respectively.
Here are 3 reasons for you to consider the Clavien-Dindo classification system in your risk management program:
1. It’s simple and validated by medical professionals
The revised 5-point grading scale has sufficient differentiation and specificity for broad applicability. There is no consideration of length of hospital stay as an indicator of severity, failure of surgery to achieve its intended purpose, and sequelae (“after-effects”). There is no ambiguous terminology such as minor or major used in the definitions of different grades.
In their 2004 article, Dr. Clavien and his collaborators presented results from a survey of 144 surgeons from Argentina, Asia, Australia, United States and Switzerland. These surgeons reported a high level of accuracy in grading 13 different scenarios and satisfaction in terms of simplicity, reproducibility and usefulness of the framework. There was no statistically significant difference based on geographical location or level of training. These are really good results indicating a high potential for consistent application by a diversity of medical professionals.
2. It has been applied broadly
In a 2009 article, Dr. Clavien and his collaborators presented findings from 5 years of experience with the Clavien-Dindo Classification using literature analysis, prospective evaluation of “difficult” cases, grading in 7 centers from each continent and perception among patients, nurses and physicians.
They found an increasing number of citations in the literature each year after the publication of their 2004 article. Moreover, these studies covered a broad range of surgical specialties such as transplantation, hepato-pancreatico-biliary (HPB – liver, pancreatic cancer, biliary tract surgery), general surgery, urology, colorectal and gynecology.
They also showed that grading of 11 complicated scenarios such as intraoperative death, post-surgery complications not caused by surgeon, death before surgical intervention, sequential development of high severity complications etc., could be accurately graded without any special considerations. Overall, about 90% agreement in grading of these complicated scenarios was reported when assessed by surgeons in 7 different centers around the world.
Finally, they reviewed the perception of severity for each grade of 30 frequent and relevant post-operative complications by physicians, nurses and patients. They found that there was a significantly higher level of perceived severity for each grade level across all groups, but particularly among patients. Patients perceived grades III and IV complications as significantly more severe than nurses and physicians. These differences in severity perceptions further highlight the value of a more objective grading scale that can be used by experts (surgeons in this case) to achieve a higher level of consistency in evaluating outcomes.
3. It’s on FDA’s radar
Recently, the Center for Devices and Radiological Health (CDRH) at the FDA issued a discussion paper on weight-loss devices, which proposes the use of a modified Clavien-Dindo Classification for benefit-risk evaluations of such devices. It is interesting to note FDA’s interest in this tool as a more objective way to evaluate the level of risk for a given amount of weight-loss outcome. Typically, FDA guidance on benefit-risk analysis has utilized a questionnaire-based approach to evaluate the type, magnitude, duration and uncertainty of benefits and risks of medical devices. The proposed approach in the discussion paper is a more objective with 5x5 grid which maps the various risk levels for each of the four weight-loss outcomes. Each grid is color coded to indicate regions of unacceptable and acceptable risk levels. This approach will significantly reduce the level of subjectivity in benefit-risk evaluation of novel medical devices and improve the predictability, consistency and transparency of the review process for all stakeholders. Medical device manufacturers, on the other hand, will need to provide more quantifiable information to facilitate this type of benefit-risk assessment during the regulatory decision process.
In conclusion, using terms such as moderate, minor or major tend to cause inconsistency and confusion in evaluating adverse events such as negative outcomes of surgical procedures. In order to improve consistency and objectivity of risk assessments, it is preferred to have a simple, yet more objective scale for severity classification. The Clavien-Dindo Classification is one such approach, which has been broadly applied in various studies, and has now under consideration for benefit-risk analysis at the FDA.
References:
ISO 14971: Medical Devices – Application of Risk Management to Medical Devices, 2010
Clavien-Dindo 2004 Paper – Classification of Surgical Complications, August 2004
Clavien-Dindo 2008 Paper – 5-year experience, August 2009
CDRH Discussion Paper – Consideration of Benefit-Risk Approaches for Weight-Loss Devices, September 2019
FDA Guidance on Benefit-Risk Determinations in Medical Device Premarket and De Novo Classifications, August 2019