FDA Wants You to Take a STeP in the Right Direction
Take Advantage of the New, Safer Technology Program (STeP) for Patient Safety
Recently, the FDA has taken 2 major steps to encourage innovation in medical devices. The first is finalizing guidance on the Breakthrough Device Program, which will enable accelerated approval of innovative medical devices that more effectively diagnose or treat life-threatening or irreversibly debilitating conditions.
The recent approval of IDX-DR, an artificial intelligence (AI) driven diagnostic system that automatically analyzes images of the retina and gives an alert if the patient has a high likelihood of developing diabetic retinopathy, is a great example. Nearly 24,000 people lose their vision due to this disease, which can be prevented through early intervention. This is an excellent case-in-point of how recent technological advancement in how artificial intelligence is shaping FDA’s thinking about regulatory approvals and compliance.
The second is a particularly exciting sign of a different approach, which promotes industry focus on patient safety. Historically, the FDA has adopted a passive approach, requiring manufacturers to report device-related injuries and malfunctions, and then taking targeted action through enforcement. Now, they are announcing a new Safer Technology Program (STeP) to support innovations that improve the safety profile of medical devices.
The core of our vision with STeP is to support important safety advancements in medical devices to help improve patients’ quality of life and advance our public health mission.
– FDA Commissioner Scott Gottlieb, M.D.
STeP seems to be more of a carrot, rather than the stick of enforcement! The idea is to accelerate market approval of devices that may not qualify for the Breakthrough Device Program, but offer substantial safety innovations that either reduce the occurrence of serious injuries or death; address a known device failure mode or common use error; or provide a significant safety advantages for users (both doctors and patients).
Safety of medical devices has been a chronic issue, although it has gained public visibility only recently due to several news reports in the media. Unlike pharmaceuticals, where blockbuster drugs attract constant scrutiny of the media and politicians, medical device industry is highly diverse and fragmented. There are thousands of medical devices in the market and the top 10 industry leaders account for less than half of the $390 billion estimated revenues in 2018. A recent report from the Associated Press has cast a bright light on this issue and the FDA is responding with a proposal to launch the STeP program.
According to a report by Associated Press, more than 1.7 million injuries and nearly 83,000 deaths suspected of being linked to medical devices have been reported to the FDA (2008-2017).
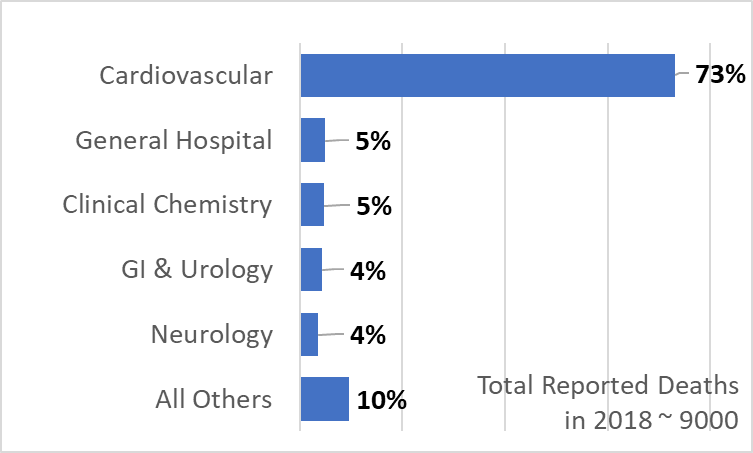
The trend of serious injuries and deaths linked to medical devices shows no signs of slowing down. We did a quick analysis of the FDA data and found that there were nearly 9,000 deaths reported in 2018. The biggest culprit is the cardiovascular category, which include devices such as stents, pacemakers, implantable defibrillators, aortic aneurysm treatment systems, etc. Patients using these devices suffer from life-threatening conditions, and FDA approval is based on a risk-benefit analysis. Still, there is a lot of opportunity to improve the safety profile of these devices to reduce the number of deaths and serious issues.
The STeP program offers manufacturers a clear incentive to focus their innovation efforts on improving patient safety. If their device can claim better safety outcomes, not only do they get faster approval, they can also gain a significant competitive advantage in the market. These devices will be of great interest to healthcare providers and payers, who are increasingly adopting an outcomes-based reimbursement model.
In order to take advantage of this opportunity, manufacturers need to improve their post-market surveillance system. No longer it is sufficient to passively monitor safety data and simply report it to the FDA and check the compliance box. A robust post-market surveillance system which proactively monitors safety signals and feeds them back to the design and development process is likely to be more effective.
I look forward to hearing more details about the STeP program! It is a great opportunity to innovate for patient safety.
References:
FDA Announcement on the STeP Program, December 2018
FDA guidance on Breakthrough Device Program, December 2018
Medical Device Safety Action Plan, November 2018
Associated Press News Story on Medical Device Safety, November 2018