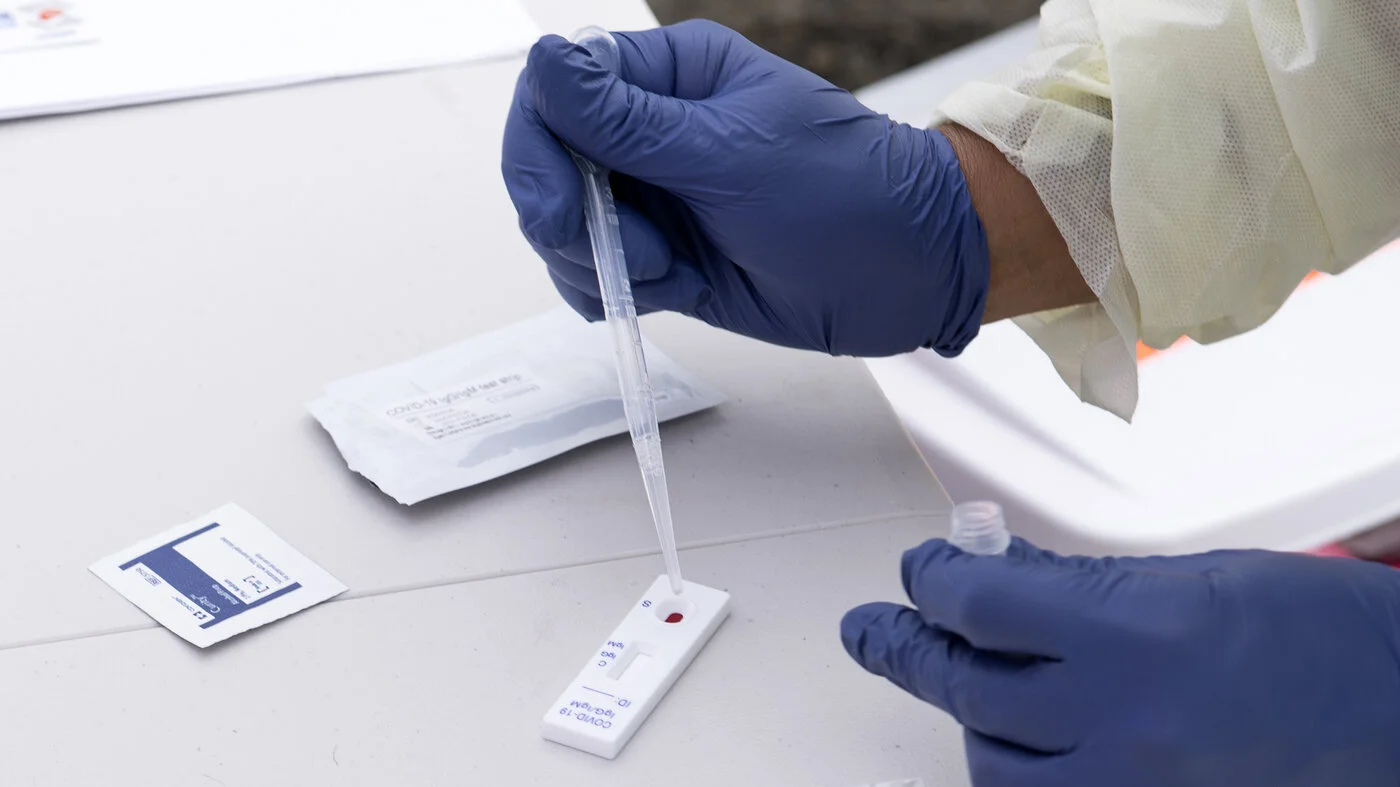
A double-blinded, placebo-controlled, randomized clinical trial provides support for FDA approval of Remdesivir for treating COVID-19. The FDA has just approved a new antiviral drug, Remdesivir from Gilead Sciences, for treating COVID-19 patients needing acute care in a hospital. This treatment was available until now under an Emergency Use Authorization (EUA), but a full FDA approval will facilitate broader use.
Read MoreThe current COVID-19 global pandemic continues to remain out of control. As we write this blog, over 60,000 new infections are being reported on a daily basis in the United States, with a cumulative total of over 4 million cases and over 150,000 deaths reported since the pandemic first began here.
Read MoreThere is a lot of conversation about testing. Antibody tests, also called serological tests, help detect the presence of antibodies in blood samples. Antibody testing, when done at large scale, can help us understand the true rates of infection, mortality rates and level of immunity in the population on a regional basis. That is why these tests are a very powerful tool to help fight this devastating disease.
Read MoreThe medical landscape is constantly changing, particularly during the COVID-19 pandemic. With so much happening, there is a lot of confusion, particularly surrounding shortages of Personal Protective Equipment (PPE) and Face Masks. The FDA is consistently updating its Guidance and policy for various Face Masks, Face Shields and Respirators as new information becomes available. In this article, we will cover Face Shields and Respirators.
Read MoreGlobal outbreak of the recent novel coronavirus needs an all-hands-on-deck approach. Here is how QA/RA professionals in the medical industry can help.
Read MoreFDA is expanding the Abbreviated 510(k) Program with a Safety and Performance-based Pathway for certain medical devices.
Read MoreFDA has issued a draft guidance on the Safer Technologies Program (STeP) for Medical Devices to promote expedited development and regulatory review of safety innovations. This is certainly a STeP in the right direction!
Read MoreRecently, there has been a lot of media attention on safety of medical devices cleared and approved in the U.S. market by the FDA.
Read MoreRecently, the FDA has taken 2 major steps to encourage innovation in medical devices. The first is finalizing guidance on the Breakthrough Device Program, which will enable accelerated approval of innovative medical devices that more effectively diagnose or treat life-threatening or irreversibly debilitating conditions.
Read MoreLast month, the FDA issued a Medical Device Enforcement and Quality Report, which provides a summary of FDA annual inspections data and a good explanation of their current approach to regulatory compliance. In providing this data and their commentary, the FDA is telling MedTech companies that they have a big stick (i.e. is the number of annual inspections has gone up in the last 10 years), but they are also offering a sweet carrot (i.e. they are taking a “risk-based” enforcement approach) to focus more on quality rather than simple compliance.
Read More