How to Avoid a Warning Letter from the FDA
A frequent reason for FDA warning letters is an inadequate corrective action plan to form-483 observations. When it is related to risk management, you need to dig deeper.
Consider this scenario – FDA issues an observation “risk analysis is inadequate” on form-483 after an inspection of design validation procedures and records. The manufacturer sends a response that they are aware of “historical inconsistencies”, and they have opened a CAPA to update all risk management files and design history files. FDA finds the response “not adequate to address the above violation” and issues a warning letter.
This is a significant escalation of non-compliance which can be avoided with a better, more effective plan, based on a deeper analysis of the immediate issue. Here, we review this case to illustrate an approach based on our experience of common challenges in risk assessment of medical devices.
Here is the cited violation from a recent FDA warning letter to a manufacturer of Class II orthopedic implants:
“Failure to establish and maintain design validation procedures to ensure proper risk analysis is completed, as required by 21 CFR 820.30(g)”.
Issue: Inconsistencies in the assignment of potential severity ratings for hazards identified during design review.
Description: The failure effect of “Compromise of the Product Sterility” is assigned different severity levels for different failure modes in the Process Failure Mode Effects and Criticality Analysis (PFMECA). In some cases, this failure effect has a severity level corresponding to the description “Necessitates Minor Medical Intervention”, while the same failure effect for another failure mode is rated with a higher severity level corresponding to the description “Results in Permanent Impairment of Body Function or Damage to Body Structure/Necessitates Surgical Intervention”. If the higher severity level is consistently applied for the failure effect of product sterility, then some of the failure modes would require further mitigation because the corresponding risk level cannot be considered as acceptable.
In citing this observation, the FDA is questioning the underlying process of risk assessment, which appears to result in inadequate risk controls that may lead to patient harm.
When a firm receives a Form-483 observation, there is a lot of pressure to respond quickly. You may be asked to come up with an aggressive plan to remediate the risk management file, design history file, and other relevant documentation. However, simply remediating documentation is not likely to address the underlying factors that lead to inconsistent assignment of severity levels in risk analysis.
FDA, however, would expect a plan for systematically investigating these underlying factors as part of a Corrective and Preventive Action (CAPA). Yes, remediation of files is required, but that is just “correction” not “corrective action”. Corrective action(s) prevent the recurrence of non-compliance by addressing the underlying factors and demonstrating effectiveness. They go beyond correcting the immediate observation found during the FDA inspection and communicated through the Form-483.
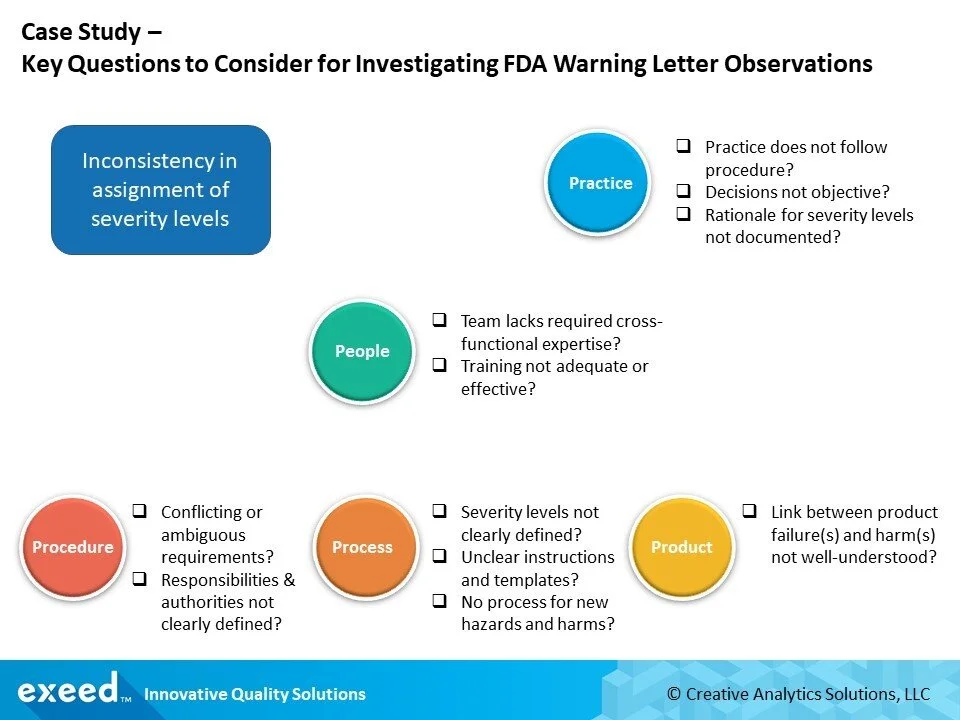
If you were responsible for leading a CAPA on this observation, how would you plan your investigation? What kind of questions should you ask?
Here is a simple framework to guide your investigation. In most cases, when inconsistencies are observed in practice, the immediate reaction is to train and re-train people to “follow procedures”. However, what may appear to be an obvious case of “practice does not follow procedure”, may require a much deeper analysis.
These questions should be only a starting point for your investigation. You can use any of the commonly known techniques such as 5-whys, Fishbone and Fault Tree Analysis in each one of the foundational areas of Procedure, Process, and Product, to probe deeper using these seed questions.
Our experience points us to the two most common challenges that contribute to inconsistencies in risk assessments of medical devices:
Severity levels are not clearly defined
It is a common practice in the industry to use qualitative terms to describe severity levels, which often have the unintended consequence of driving value-driven judgments based on individual risk perception. Terms such as Minor, Major, Critical, Catastrophic, etc. trigger mental images associated with prior experiences and awareness at an individual level. Research has shown that individual evaluation of risk is highly influenced by affect, which is the specific feeling of goodness or badness experienced with or without conscious awareness. As a result, individuals interpret and evaluate these qualitative terms differently even when seemingly clear, consistent definitions are provided. What is Minor to one person may be Major, or even Catastrophic, to another.
An alternate approach, especially suitable for medical devices that require surgery, is the Clavien-Dindo Severity Classification System. It is a 5-point grading scale which is highly precise in its definitions from a medical terminology viewpoint, and more accurately relates to various patient outcomes. This simple framework is increasingly becoming more popular across the world, and even the FDA is considering its application for benefit-risk assessments of certain medical devices.
The key point is to be aware of the influence of individual perceptions and develop a system of checks and balances in the risk assessment process. It is not always possible to precisely define severity levels, but every effort should be made to reduce ambiguity in their definition.
Link between product failure(s) and harm(s) is not well understood
It is quite reasonable that different failure modes may lead to situations where the level of potential harm is different depending on the sequence of events triggered by the effect of “compromised sterility”. Sterility of a medical device is required to reduce the risk of infection; however, an infection can result from many other sources before, during and after the procedure. Further, an infection can be localized to the site of the medical device or spread to other areas triggering a life-threatening medical emergency. Some infections may be treated with antibiotics, while some others may require a second surgical intervention. There may be additional complications due to the patient’s own health history and ability to recover. Timeliness and the extent of medical intervention may be critical factors affecting death or survival. Even if death doesn’t occur, the patient may suffer from a permanent disability which otherwise would not happen if the medical device was not implanted in the first place.
The key point is that there are many different pathways through which a patient may experience harm, and each pathway may lead to a different type of harm, or the same harm with different levels of severity. There are a lot of factors, not just an effect of one or more device failure modes, which affect the type and severity of harms.
The assignment of severity level to a failure effect can be influenced by one or more of such considerations. If the link between hazards (example: product failure) and harms (example: permanent disability caused by a major surgical intervention due to a device-related infection) is not clearly defined, it is possible that different people may rate the severity level of the same effect differently depending on the circumstances they envision resulting from a specific product failure. Additionally, the use of risk analysis tools such as a PFMECA or a PFMEA does not facilitate a clear linkage between hazards and harms. We will be writing more about this topic in future articles.
In conclusion, it is quite natural to want a quick, aggressive remediation plan after receiving a Form-483 observation, or a follow-up Warning Letter. However, it is important to take a step back and consider several key questions at a deeper level so you can develop a better, more effective, corrective action plan. This is particularly important for observations related to risk assessments, such as inconsistencies in the assignment of severity levels, which tend to be related to the underlying system and process challenges.
Recent actions from the FDA indicate a willingness to work with violative firms to help develop an appropriate plan of corrective action that can avoid an escalation to a warning letter. FDA has formalized this approach by issuing a draft guidance, Nonbinding Feedback After Certain Food and Drug Administration Inspections of Device Establishments, as a potential mechanism for this purpose.
One of the main reasons why FDA issues a Warning Letter is an unsatisfactory response to a Form-483 observation. Don’t let that happen under your watch!
References:
FDA Warning Letter to a manufacturer of an orthopedic implant CMS #558176, issued August 2018
When a Warning Fails, May 2019
FDA Draft Guidance – Nonbinding Feedback after FDA Inspections, February 2019
ISO 14971: Medical Devices – Application of Risk Management to Medical Devices, September 2010